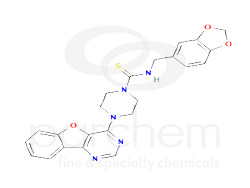
N-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide

Get a Quick Quote Now
Select Packaging
Product
N-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide
CAS
850879-09-3
Formula
C23H21N5O3S
Product Description
N-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide, also known as Amuvatinib, is a chemical compound with potential applications in pharmaceutical development. It features a complex molecular structure and is characterized by its unique combination of benzodioxole and benzofuro-pyrimidine moieties.
Need a quote for N-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide?
Please fill out the RFQ form, and one of our representatives will contact you as soon as possible.
Typical Product Specifications
Canonicalized Compound
1
Compound Complexity
678
Hydrogen Bond Acceptor Count
7
Hydrogen Bond Donor Count
1
Rotatable Bond Count
3
Allowed IUPAC Name
N-(1,3-benzodioxol-5-ylmethyl)-4-(benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide
CAS-like Style IUPAC Name
N-(1,3-benzodioxol-5-ylmethyl)-4-(4-benzofuro[3,2-d]pyrimidinyl)-1-piperazinecarbothioamide
Markup IUPAC Name
<I>N</I>-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide
Preferred IUPAC Name
N-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide
Systematic IUPAC Name
N-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide
Traditional IUPAC Name
4-(benzofuro[3,2-d]pyrimidin-4-yl)-N-piperonyl-piperazine-1-carbothioamide
Standard InChI
InChI=1S/C23H21N5O3S/c32-23(24-12-15-5-6-18-19(11-15)30-14-29-18)28-9-7-27(8-10-28)22-21-20(25-13-26-22)16-3-1-2-4-17(16)31-21/h1-6,11,13H,7-10,12,14H2,(H,24,32)
Standard InChIKey
FOFDIMHVKGYHRU-UHFFFAOYSA-N
XLogP3-AA Log P
3.5
Exact Mass
447.13651072
Molecular Weight
447.5
Canonical SMILES
C1CN(CCN1C2=NC=NC3=C2OC4=CC=CC=C43)C(=S)NCC5=CC6=C(C=C5)OCO6
Isomeric SMILES
C1CN(CCN1C2=NC=NC3=C2OC4=CC=CC=C43)C(=S)NCC5=CC6=C(C=C5)OCO6
Polar Surface Area Topological
108
MonoIsotopic Weight
447.13651072
Chemical Structure
Class
Uses of N-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide
Amuvatinib is primarily used in the pharmaceutical industry as an active pharmaceutical ingredient (API) for the development of targeted therapies, particularly in oncology. Its unique chemical properties make it a candidate for research into treatments for various cancers, potentially acting as a kinase inhibitor.
Industries that use N-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide
- Pharmaceutical - The pharmaceutical industry utilizes Amuvatinib in drug formulation and development, focusing on its efficacy in treating specific types of cancer.
- Biotechnology - Biotechnology firms may explore Amuvatinib for its potential in developing innovative therapies and conducting clinical trials.
- Research and Development - Research institutions may use Amuvatinib in studies aimed at understanding its mechanisms of action and potential therapeutic applications.
Related Products
- Complementary Products
- Alternative Products
Contact Us
N-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide

Product
N-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide
CAS
850879-09-3
Formula
C23H21N5O3S
Product Description
N-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide, also known as Amuvatinib, is a chemical compound with potential applications in pharmaceutical development. It features a complex molecular structure and is characterized by its unique combination of benzodioxole and benzofuro-pyrimidine moieties.
Need a quote for N-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide?
Please fill out the RFQ form, and one of our representatives will contact you as soon as possible.
Typical Product Specifications
Canonicalized Compound
1
Compound Complexity
678
Hydrogen Bond Acceptor Count
7
Hydrogen Bond Donor Count
1
Rotatable Bond Count
3
Allowed IUPAC Name
N-(1,3-benzodioxol-5-ylmethyl)-4-(benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide
CAS-like Style IUPAC Name
N-(1,3-benzodioxol-5-ylmethyl)-4-(4-benzofuro[3,2-d]pyrimidinyl)-1-piperazinecarbothioamide
Markup IUPAC Name
<I>N</I>-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide
Preferred IUPAC Name
N-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide
Systematic IUPAC Name
N-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide
Traditional IUPAC Name
4-(benzofuro[3,2-d]pyrimidin-4-yl)-N-piperonyl-piperazine-1-carbothioamide
Standard InChI
InChI=1S/C23H21N5O3S/c32-23(24-12-15-5-6-18-19(11-15)30-14-29-18)28-9-7-27(8-10-28)22-21-20(25-13-26-22)16-3-1-2-4-17(16)31-21/h1-6,11,13H,7-10,12,14H2,(H,24,32)
Standard InChIKey
FOFDIMHVKGYHRU-UHFFFAOYSA-N
XLogP3-AA Log P
3.5
Exact Mass
447.13651072
Molecular Weight
447.5
Canonical SMILES
C1CN(CCN1C2=NC=NC3=C2OC4=CC=CC=C43)C(=S)NCC5=CC6=C(C=C5)OCO6
Isomeric SMILES
C1CN(CCN1C2=NC=NC3=C2OC4=CC=CC=C43)C(=S)NCC5=CC6=C(C=C5)OCO6
Polar Surface Area Topological
108
MonoIsotopic Weight
447.13651072
Chemical Structure

Class
Uses of N-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide
Amuvatinib is primarily used in the pharmaceutical industry as an active pharmaceutical ingredient (API) for the development of targeted therapies, particularly in oncology. Its unique chemical properties make it a candidate for research into treatments for various cancers, potentially acting as a kinase inhibitor.
Industries that use N-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide
- Pharmaceutical - The pharmaceutical industry utilizes Amuvatinib in drug formulation and development, focusing on its efficacy in treating specific types of cancer.
- Biotechnology - Biotechnology firms may explore Amuvatinib for its potential in developing innovative therapies and conducting clinical trials.
- Research and Development - Research institutions may use Amuvatinib in studies aimed at understanding its mechanisms of action and potential therapeutic applications.
Related Products
- Complementary Products
- Alternative Products
Contact Us


